Our Science
Nitric oxide in health and disease
Nitric oxide (NO) is a remarkable gas naturally produced by the body, playing a crucial role in improving blood flow and oxygen delivery to tissues by dilating blood vessels. In young and healthy individuals, the body’s abundant NO production acts as one of several protective shields for the airways and blood vessels, safeguarding them against diseases such as infections.
However, as we age, our natural NO production declines, leaving us vulnerable to ailments affecting the airways and pulmonary vascular system. Also younger patients can suffer from NO deficiency, hindering their ability to combat infections or arterial blockages.
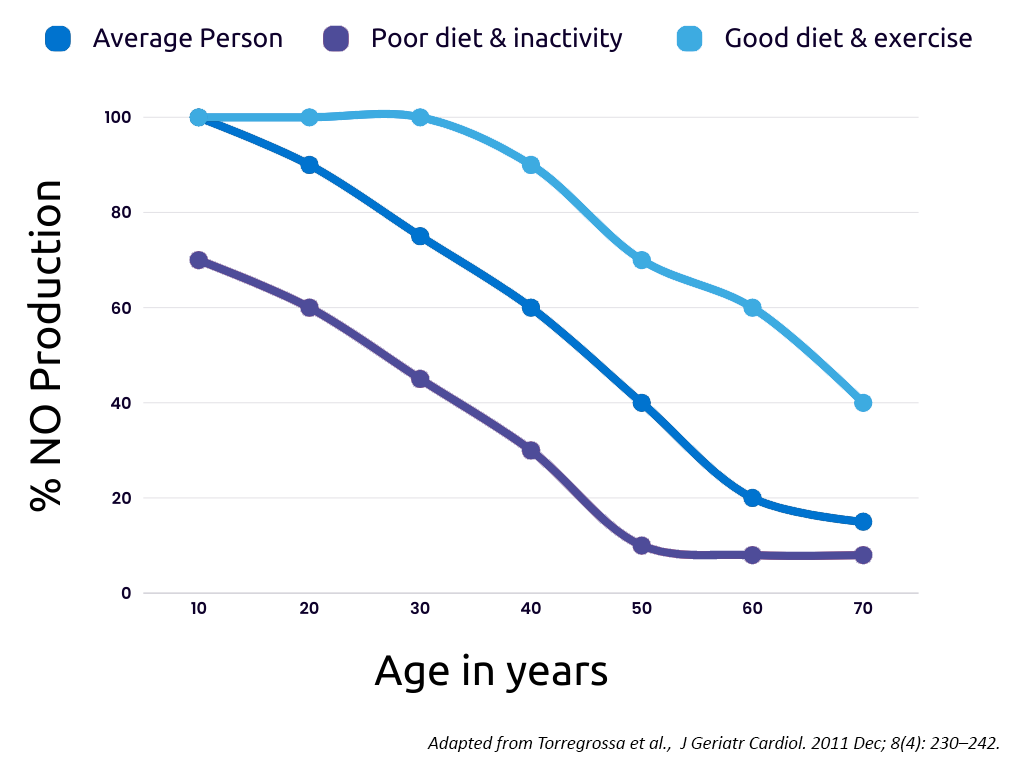
The consequences of low NO levels can be severe, leading to damage to the heart and other vital organs, and in critical situations, it can become life-threatening, requiring intensive care measures and respiratory assistance for the patient’s survival.
NO-based drugs
To counteract the detrimental effects of low NO-levels, NO-based drugs have been in clinical use for over a century due to their protective and beneficial effects.
NO-based drugs can be divided into two groups, NO-donors or inhaled NO. NO-donors are drugs that releases nitric oxide (NO) under specific conditions, such as in contact with blood. Nitroglycerin, one of the earliest NO-donors, has been employed to treat cardiac disease since the 19th century and is still utilized today. However, the NO-based treatments available today have limitations.
The currently available NO-donors have a long half-life, resulting in difficulties in rapid discontinuation of treatment and release of NO throughout the entire circulatory system, causing a dangerous reduction in systemic blood pressure. In addition, some NO-donors induce tolerance development over time.
Inhaled NO on the other hand, is employed to assist premature infants with high blood pressure in their lungs and difficulties in oxygenation. Inhaled NO is also used to protect the heart during heart surgery when high blood pressure in the lung blood circulation arises. Unfortunately, inhaled NO acts only locally in the lungs, and does not reach all relevant vessels in need of dilatation and therefore lack efficacy in many conditions.
Introducing Supernitro: A paradigm shift in NO-delivery technology
Supernitro, Attgeno’s lead substance, marks a groundbreaking advancement in NO-delivery. It is the first and only NO-donor infused into the blood that enables targeted, organ-specific delivery of NO. This unique capability opens new possibilities for treating life-threatening conditions, such as acute high blood pressure in the lungs, by administering Supernitro intravenously. The targeted NO-delivery attainable with Supernitro suggests a safer alternative compared to other NO-donors, because the risk of dangerous systemic blood pressure drops is minimized.
Moreover, by utilizing intra-arterial administration, targeting organs other than the lung where vessel dilation is desired becomes feasible. This paves the way for NO-treatments in conditions such as arterial occlusions in stroke, myocardial infarction, and solid tumors, as well as those where other potential beneficial effects of NO, including antimicrobial properties, could be harnessed for the treatment of various pathogens.
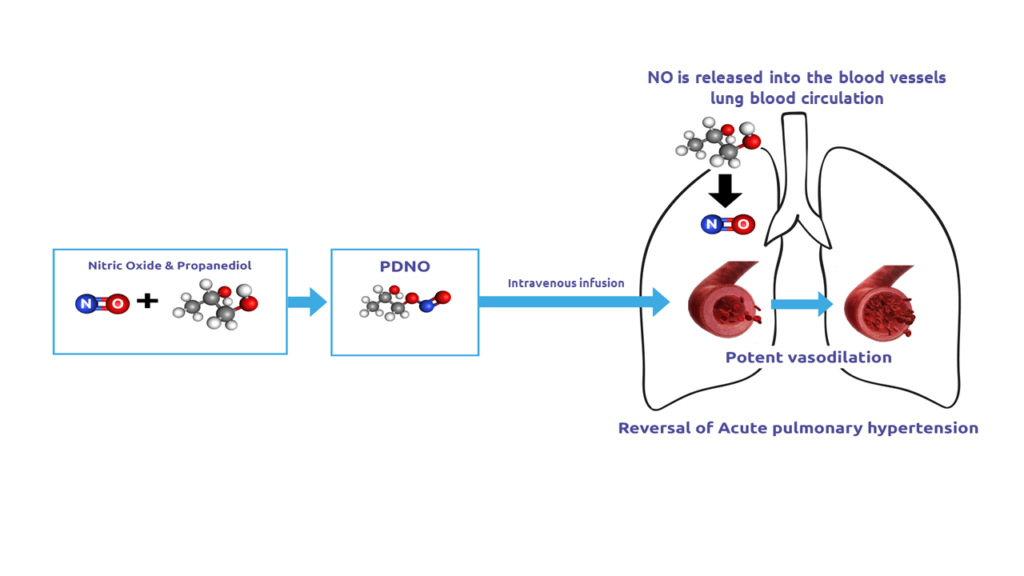
Supernitro’s mechanism of Action
Due to it’s ultra-short half-life Supernitro (PDNO) after being infused into the blood rapidly releases NO, which acts locally and is then immediately metabolized to nitrite and nitrate. Only propanediol (PD) will remain in the system and with the doses of Supernitro needed for effective vasodilation, the exposure for PD is well below recommended limits. When infused intravenously, Supernitro has lung-selective action since the bulk of the NO contained in the drug will be released, and have its action, in the lung-blood circulation. Targeted delivery to other organs could be accomplished by intra-arterial infusion.
Safety redefined and a future without tolerance development
Supernitro’s unique safety profile is built upon its construction using propanediol (PD), a widely used excipient already used in infusion-administered drugs, to which NO is attached as an organic nitrite.
As NO is also endogenously produced in the body, the risk of unexpected immunological reactions is negligible, and PD’s safety profile is well-characterized. Supernitro’s extremely short half-life upon administration (<10 s) ensures that any unwanted effects can be rapidly terminated by halting the infusion, which is particularly valuable when treating vulnerable patients in intensive care settings.
In contrast to many other NO-donors where effect diminishes over time, Supernitro has shown promising results indicating little or no tolerance development. Additionally, some NO-donors are known to increase the tissue content of radical oxygen species (ROS), potentially causing increased tissue damage. Thanks to Supernitro’s controlled NO liberation, the risk of this phenomenon is greatly reduced.


